In 1859, French physician Gaston Planté pioneered the commercial use of lead acid — the first rechargeable battery. In recent years, lead-acid batteries have been optimized in numerous applications for its reliability and inexpensive cost-per-watt base but there remain certain new shortcomings facing the lead-acid battery manufacturers —an increase in the amount of faulty modes.
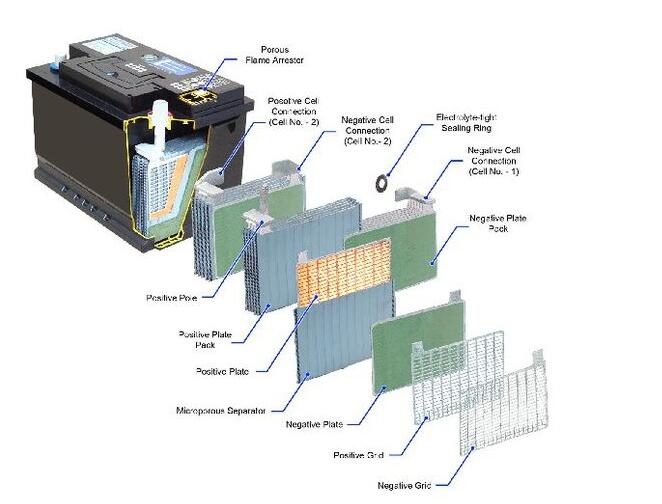
Image 1. Components of Lead Acid Battery (Source thebatteryclinic.co.nz)
●Batteries with flooded or excess electrolyte.
●Low-maintenance lead-acid batteries with a large excess of electrolyte.
●Batteries with immobilized electrolyte and a pressure-sensitive valve usually referred to as absorptive glass-microfibre (AGM) valve-regulated lead-acid (VRLA) batteries.
Advantage | Disadvantages |
●Inexpensive and simple to manufacture; low cost per watt-hour ●Low self-discharge; lowest among rechargeable batteries ●High specific power, capable of high discharge currents ●Good low and high temperature performance | ●Low specific energy; poor weight-to-energy ratio ●Slow charge; fully saturated charge takes 14-16 hours ●Must be stored in charged condition to prevent sulfation ●Limited cycle life; repeated deep-cycling reduces battery life ●Flooded version requires watering ●Transportation restrictions on the flooded type ●Not environmentally friendly |
Table 1. Advantages and Limitations of Lead Acid Batteries (Retrieved from Battery University)
(Read also: The Best Start-stop Car Battery)
Lithium-ion began to surface in the 1970s, yet these immediate batteries are not rechargeable. Thus in the 1980s, rechargeable versions were manufactured. Nowadays, it's the most common battery in commercial use. Lithium is lightweight in contrast to other metals and the highest density per kilogram. Therefore, lithium is the most ideal for batteries since it contains significant amounts of energy and has the greatest electrochemical potential.
Inside a Lithium-ion battery there is a negative and positive electrode. The cathode is built from lithium cobalt oxide (LiCoO2). The anode is reared of carbon and both anode and cathodes are suppressed in an electrolyte. The battery is equipped with a separator between cathode and anode then the Lithium-ions from the cathode roams through the electrotype and separator to the anode.
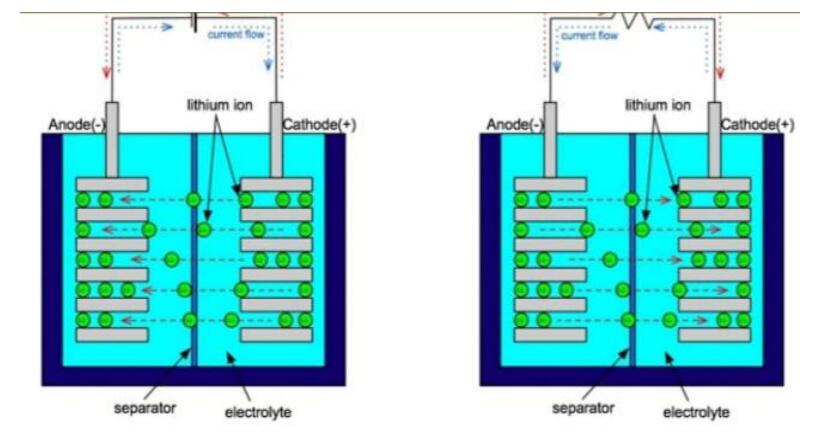
Image 2. Retrieved from physicsandsocietybc.wordpress.com
According to Kejie Zhao's paper on “Fracture Of Electrodes In Lithium-Ion Batteries Caused By Fast Charging.” Journal Of Applied Physics (2010), The electrons from the cathode travel through a metallic wire outside of the battery and amidst this process 3.7 volts are produced in the cell. In its recharging state all of this is reversed and the lithium ions are sent back to the cathode.
(Read also: Lithium Iron Phosphate VS. Lithium-Ion: Differences and Advantages)
The development dates back to 1996. The University of Texas discovered phosphate as cathode material for rechargeable lithium batteries. Li-phosphate offers good electrochemical performance with low resistance. This is made possible with nano-scale phosphate cathode material (Battery University, 2021). Its fundamental benefits are good thermal stability, extensive safety, tolerance, high current rating and long-term cycle life.
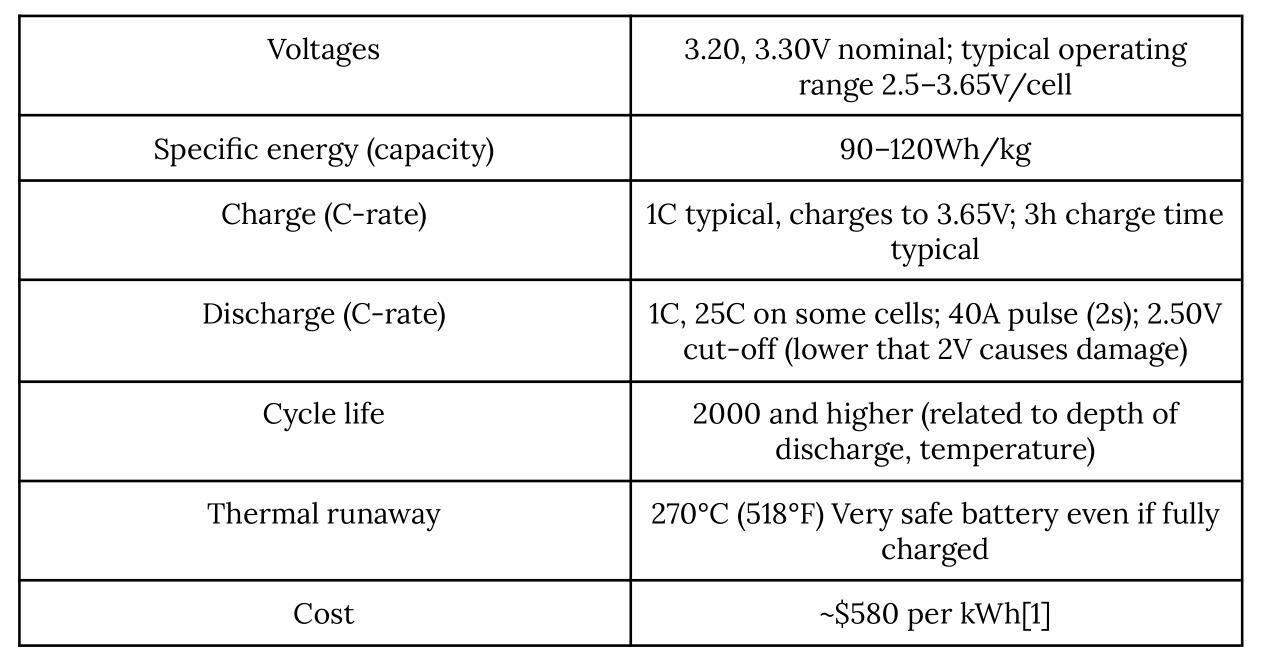
Table 2. Characteristics of Lithium-ion Phosphate (Retrieved from Battery University)
Even when hoarded for a year inside the storage space, Lithium-ion Phosphates batteries remain to have a similar energy density for its slow decline of energy density. Lithium-ion Phosphate batteries are way more dependable and safer than standardized Lithium-ion batteries. It does not decompose at high thermal operations and it is harder to ignite amidst charging and discharging.
Previous: Elevating Electronics: Multilayer PCBs with High Copper Thickness and Aluminum Base by sqpcb.com
Next: LED Totem
Copyright:@2020-2021
Comments Please sign in or sign up to post.
0
0 of 500 characters used